The Ultraviolet Catastrophe
by damnedhippie
Part 2 in a series about quantum mechanics. Read Part 1 here.
Nearing the end of the 19th century, it was the opinion of many that the science of physics was nearing completion, that physicists only had to fill in a few details, and then they would be out of a job. However, at least one of those assumedly trivial details turned out to be rather significant. Regular every-day heated objects don’t cause sunburns. This was the first indication that classical physics did not sufficiently explain the universe.
Wait, what? Why would kitties, car engines, or hot soup cause sunburns? Because classical physics predicts that any object giving off heat should also give off a huge amount of UV rays.
Light
What we feel as heat is really just another form of light. It is not light we can see obviously. In other words, the light we can see is just a small part of the spectrum of electromagnetic radiation.
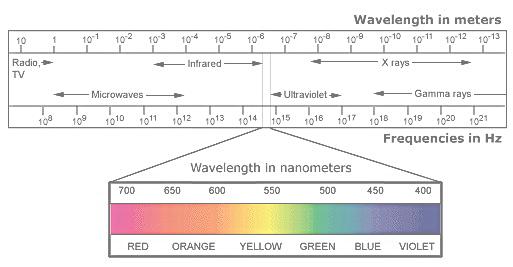
The color of light is determined by its wavelength. The wavelength of light decreases as the energy and frequency of the light increases. Infrared light, which we can’t see, but we feel as heat, has a longer wavelength, lower frequency, and less energy than the light that we can see. Ultraviolet light has more energy, higher frequency and shorter wavelength than visible light.
Blackbody Radiation
An object at any temperature gives off light (electromagnetic radiation). The wavelength at which an object emits the most light depends only on its temperature. The hotter the object, the shorter the wavelength. This is how we calculate the temperature of stars.
So, if anything with a temperature gives off light, why doesn’t everything glow? Again, it is not light that we can see with our naked eyes. Most things are so cool, they give off light in frequencies smaller than what our eyes can see.
A black body is a fictional object (like a frictionless surface or a perfect insulator) invented by physicists that absorbs and emits all wavelengths of light. It is used to model how much light is emitted by an object at each frequency as the object is heated.
The Catastrophe
The problem is, physics before quantum mechanics calculates that the intensity of light from a blackbody doesn’t peak at a particular color at all, but instead just keeps going up at higher frequencies of light. In other words, an object at any temperature would radiate copious amounts of ultraviolet radiation- the high energy light that causes sunburns and skin cancer. Hence the name, Ultraviolet Catastrophe.
This is a catastrophe for at least two reasons. First, it utterly fails to align with what happens in the real world. Real objects do not cause sunburns unless they are as hot as, well, the sun.
Second, it violates the law of conservation of energy- the capacity to do work cannot be created or destroyed. It can only change form. So, imagine a scientist takes a rock and sets it on a hot plate. A finite amount of electrical energy goes into the hot plate, which is transformed into heat, which is transferred to the rock by conduction (the rock touching the hot plate). The energy that started out as electrical energy is now heat energy in the rock. The rock, now being a heated object, gives off electromagnetic radiation.
If the rock follows the old laws of physics, it would radiate an infinite amount of high-energy ultraviolet radiation. So, in exchange for a finite amount of electrical energy, our scientist would get an infinite amount of light energy. Set in front of it a solar-powered mechanical device that generates electricity to run the hot plate, and you have a perpetual motion machine.
Perpetual motion machines do not exist. They violate the law of conservation of energy.
Quantize It!
In 1900, a superhero named Max Planck saved physics from the ultraviolet catastrophe. Light is released when a charged particle (usually an electron) loses kinetic energy. Planck simply postulated that the energy contained within an electron couldn’t be just any number, but instead was proportional to its frequency of oscillation (orbit around the nucleus) multiplied by a whole number (1, 2, 3, 4 . . . ):
Energy = (whole number) x (frequency of oscillation) x (planck constant)
The planck constant is a number (approximately 0.00000000000000000000000000000000000662 joule seconds) represented in physics by the letter h.
The effect of this one simple adjustment solved the ultraviolet catastrophe. The mathematical model derived from this postulate accurately predicts the blackbody radiation given off by real heated objects. It also kicked off the next big thing in physics, a field of science once thought to be nearing completion. It turns out there was a huge devil in that detail.
Up next: The Photoelectric Effect




Why a rock will transmit electrical energy to a hot plate????
Please explain..
The hot plate heats the rock, he is using it as an example to show that if you heat an object, it will not give off an infinite amount of energy, but will only give of the energy put into it (once it reaches thermal equilibrium). Hopefully my comment helps.
and to the hippie, great article!
When some one searches for his required thing, therefore he/she wishes to be available that in detail, therefore
that thing is maintained over here.
Everything is very open with a really clear clarification of the
issues. It was really informative. Your website is very
helpful. Many thanks for sharing!